Biography
Why do some individuals suffer more than others from infection?
I seek to understand why individuals within a given species respond differently to environmental challenges, especially from parasitic organisms. I apply concepts from evolutionary biology to model invertebrates (e.g., Drosophila melanogaster, Daphnia magna), and combine empirical approaches such as controlled infections and functional genetics with statistical modelling, genomics, and transcriptomics to study the role of host, and parasite, evolution in this variation in disease outcome.
Download my CV. Publication list with summaries.
Interests
- Evolutionary parasitology
- Infectious diseases
- Sexual dimorphism
- Genetic basis of quantitative traits using GWAS
- Resistance vs disease tolerance
- Within-host dynamics and evolution
Education
PhD student in Evolutionary Parasitology, 2011
Dieter Ebert's lab, Basel University, Switzerland
Master in Ecology and Evolutionary Biology, 2006
University of Montpellier II, France
BSc in Organismal Biology, 2005
University of Montpellier II, France
Projects

Sexual dimorphism of diseases
Most infectious diseases have a sexually dimorphic outcome. We investigate the reasons for this not only with a host-centered view but also by considering that pathogens adapt to the most commonly encountered sex.

Host manipulation by parasites
One of the parasite strategy to increase its transmission is to manipulates its host. I started science by studying such strategy.
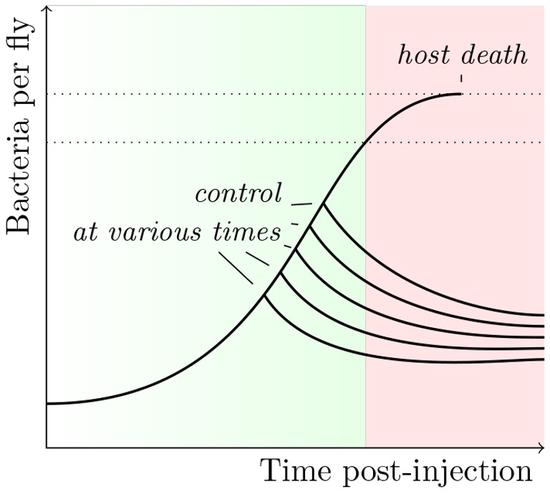
Within-host dynamics and disease outcomes
Infection outcome depends on the success of the parasite within its host. With theoretical and empirical approaches, we study what influences pathogen dynamics and its implication on symptoms.

Sexual selection in parthenogenetic species
Parthenogenetic species produce mainly daughter without males. We study the selection during the rare event of sexual reproduction.
Mechanism host-parasite coevolution
Parasites need to go through several infection steps to be successfull. We study how the evolution at each step shapes host-parasite coevolution.
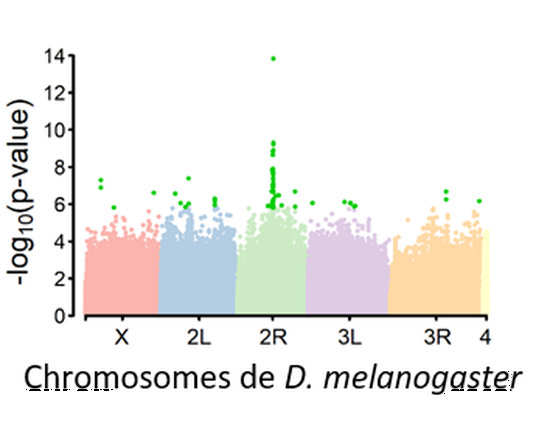
Genetic basis of quantitative traits
Genetic variation is the raw material for evolution. We pertain to identify, using GWAS and the Drosophila Reference Genetic Panel (DGRP), the genetic basis of various quantitative traits (e.g. phenotypic plasticity, insecticide resistance).

Lyme disease in seabirds
Lyme disease is a major threat in many countries. The bacteria responsible for it is mainly transmitted by ticks feeding on mammals. We studied the role of seabirds as reservoir.
Featured publications
Diet composition plastically resizes the Drosophila midgut by affecting cell gain and loss, stem cell-niche coupling and enterocyte size.
We studied the phenotypic plasticity of Drosophila gut in response to glucose level in diet.
Additive and non‐additive effects of day and night temperatures on thermally plastic traits in a model for adaptive seasonal plasticity.
We tested the effects of circadian temperature fluctuations on a series of thermal plasticity traits in a model of adaptive seasonal plasticity, the Bicyclus anynana butterfly.
Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in Drosophila melanogaster.
A central problem with biomedicine is to understand why two individuals exposed to seemingly identical infections may have radically different clinical outcomes. Using the Drosophila melanogaster model, we analyse in depth, both through functional genetics and mathematical modelling, the main determinants that underlie the stochastic outcome of infection.
Host sexual dimorphism and parasite adaptation.
In this “essay” we propose for the first time the idea that the sexual dimorphism of diseases may be the result of the specific adaptation of parasites to the sex of their host. Similarly, as organisms adapt to the environment to which they are most frequently exposed, parasites can adapt to the sex they encounter most frequently (e.g., either because males and females are exposed differently, or because one sex is more easily infected than another due to immune differences). As a result, parasites behave differently depending on the sex they infect.
Publications
Five most recents
Diet composition plastically resizes the Drosophila midgut by affecting cell gain and loss, stem cell-niche coupling and enterocyte size.
We studied the phenotypic plasticity of Drosophila gut in response to glucose level in diet.
Additive and non‐additive effects of day and night temperatures on thermally plastic traits in a model for adaptive seasonal plasticity.
We tested the effects of circadian temperature fluctuations on a series of thermal plasticity traits in a model of adaptive seasonal plasticity, the Bicyclus anynana butterfly.
Step-specific adaptation and trade-off over the course of an infection by GASP-mutation small colony variants
Within-host bacterial adaptations are generally focused on antibiotic resistance, rarely on the adaptation to the environment given by the host, and the potential trade-off hindering adaptations to each step of the infection are rarely considered. Using Drosophila melanogaster as host and the bacteria Xenorhabdus nematophila, we studied those trade-offs that are key to understand intra-host evolution, and thus the dynamics of the infection.
Sex and hatching order modulate the association between MHC-diversity and fitness in early-life stages of a wild seabird.
We found that, in black-legged kittiwake (Rissa tridactyla) chicks, associations between MHC class-II diversity and fitness vary with sex and hatching order.
An alternative route of bacterial infection is associated with a polymorphism at an alternative resistance locus.
To understand the mechanisms of antagonistic coevolution, it is crucial to identify the genetics of parasite resistance. Using QTL approach, we discovered a second P. ramosa attachment site and a novel host-resistance locus, with implications for both for the coevolutionary dynamics (e.g., Red Queen and the role of recombination), and for the evolution and epidemiology of the infection process.
Contact
- david.duneau@gmail.com
- Kings Buildings, Charlotte Auerbach Road
EH9 3FL Edinburgh - Ashworth Laboratories
- DDuneau









